- English
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
Gilteritinib Fumarate
2023-12-02
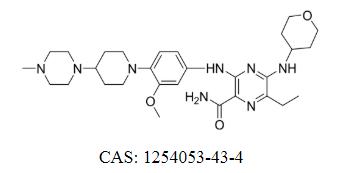
Gilteritinib Fumarate Cas: 1254053-43-4
1. Background
Targeted drugs for Gillette's (Gilteritinib) for acute myeloid leukemia patients live longer, better recently published in the New England journal of medicine of a large clinical trial results show that: compared with chemotherapy, the use of targeted drugs for Gillette's (Gilteritinib, commodity name Xospata) treatment, can improve some the survival rate of patients with acute myelogenous leukemia (AML).
The results of the new trial are encouraging. The 371 patients enrolled in the trial were AML patients with a specific mutation of the FLT3 gene who had previously been treated but later relapsed or did not respond to treatment (recurrent/refractory). They were randomly assigned to either Gilteritinib treatment or standard chemotherapy.
The results showed that patients treated for accepting gillett, Gilteritinib not only live longer than in patients undergoing chemotherapy (median overall survival time was 9.3 months to 5.6 months), and are more likely to achieve complete remission, white blood cell count returned to normal level in whole or in part (Gilteritinib treated patients (34%), 15%) in patients undergoing chemotherapy.
2. Presentation
Gilteritinib Fumarate, developed by Astellas, was approved by the Japanese Pharmaceutical Medical Devices And Devices Integrated Agency (PMDA) on 21 September 2018, subsequently approved by the US Food and Drug Administration (FDA) on 28 November 2018, and by the European Drug Administration (EMA) on 24 October 2019 under the trade name Xospata®. Gilteritinib has received fast-track and orphan drug status from the FDA.
Gilteritinib Fumarate is a FLT3/AXL inhibitor and Xospata® is approved for the treatment of recurrent or refractory acute myeloid leukemia with FLT3 mutation positive.
Xospata® is an oral tablet containing 40 mg of Gilteritinib. The recommended dose is 120 mg once a day. Increase or decrease the dose according to the patient's condition, but should not exceed 200 mg per day.
3. Target spot
AXL; FLT3
4. Mechanism of action
AXL receptor inhibitors; FLT3 inhibitors
5. Indications
Acute myelogenous leukemia
6. Development stage
Approved for marketing on 21 September 2018
7. R&D Company
Astellas
8. Route of Synthesis
8.1. The original route
8.2. Our route(optimized)- Better stability and higher yield
8.3. Ros of KSM [CAS 2043020-03-5]
8.4. Brief Manufacturing Process [CAS 2043020-03-5]
Step 1:
To a suspension of NaOH was added ethyl 3-oxopentanoate in one-portion, and then the reaction mixture was stirred at r.t. A solution of NaNO2 in water was added, and then H2SO4 was added dropwise. A solution of NaOH was added dropwise, and the resulting mixture was extracted with MTBE. The combined organic layers were washed with brine, dried over Na2SO4, filtered and concentrated to give product (E)-2-oxobutanal oxime.
Step 2:
A suspension of (E)-2-oxobutanal oxime and Aminomalononitrile p-Toluenesulfonic Acid in IPA ) was stirred at r.t under argon. After it was checked by TLC the reaction mixture was filtered, the cake was washed with IPA and water, and dried to afford 2-amino-3-cyano-5-ethylpyrazine 1-oxide.
Step 3:
To a suspension of 2-amino-3-cyano-5-ethylpyrazine 1-oxide in anhydrous DMF was added POCl3 at 0oC. The resulting mixture was stirred at 80oC. After it was checked by TLC the reaction mixture was added into ice/water slowly and extracted with MTBE. The combined organic layers were washed with brine, dried over Na2SO4, filtered and concentrated to afford 3-amino-5-chloro-6-ethylpyrazine-2-carbonitrile.
Step4:
To a suspension of t-BuONO and CuBr2 in DMF was added a solution of 3-amino-5-chloro-6-ethylpyrazine-2-carbonitrile in DMF dropwise. After it was checked by TLC the reaction mixture was cooled to r.t, and then poured into ice/water, extracted with MTBE. The combined organic layers were washed with brine, dried over Na2SO4, filtered and concentrated to give crude which was purified by chromatography to afford 3-bromo-5-chloro-6-ethylpyrazine-2-carbonitrile.
9. List of intermediates we can supply
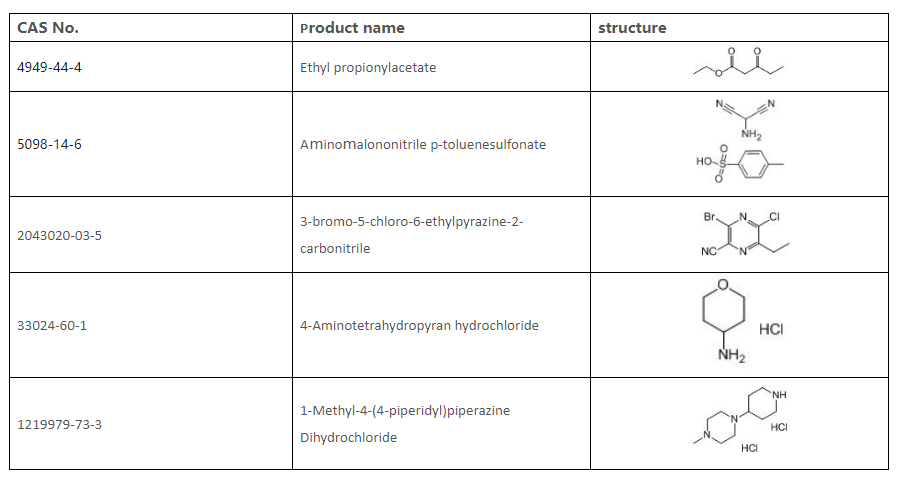
Sandoo Pharmaceutica is a professional pharmaceutical intermediates manufacturer. We provide good quality Gilteritinib Fumarate Cas: 1254053-43-4. Welcome and we look forward to your inquiry!